Potassium Dichromate and Potassium Permanganate
Potassium Dichromate and Potassium Permanganate: Overview
This topic covers concepts, such as, Potassium Dichromate,Preparation of Potassium Dichromate,Physical Properties of Potassium Dichromate etc.
Important Questions on Potassium Dichromate and Potassium Permanganate
A compound which is a strong oxidizing agent and has orange coloured crystal. It is used in the preparation of azo compounds. Identify the compound:
On oxidation of by in neutral aqueous medium, the oxidation number of S would change from:
On complete oxidation of in acidic medium, the oxidation state of Cr will change from:
The number of moles of that will be needed to react with one mole of sulphite ion in acidic solution is:
In the scheme given below, and , respectively, are
In alkaline medium, the reduction of permanganate anion involves a gain of ____________ electrons.
Given below are two statements:
Statement I : Aqueous solution of is preferred as a primary standard in volumetric analysis over aqueous solution.
Statement II : has a higher solubility in water than . In the light of the above statements, choose the correct answer from the options given below:
How many electrons are gained by in strongly alkaline medium.
During bleeding from a cut is used to stop bleeding as:
Read the following two statements.
Statement I: Potassium dichromate is used in volumetric analysis.
Statement II: is more soluble in water than
Oxidation state of in changes by three units in which medium ?
Write the pyrolysis product of ?
Explain oxidising nature of Potassium Permanganate in acidic medium?
Name the reagents used in the following reaction:
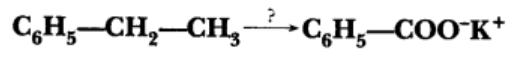
Standard electrode potential data are useful for understanding the suitability of an oxidant in a redox Titration. Some half cell reactions and their standard potential are given below:
Identify the only incorrect statement regarding the quantitative estimation of aqueous
When to use and when to use in oxidation reaction?
Write the net ionic equation for the reaction of potassium dichromate with sodium sulphite in an acid solution to give chromium ion and the sulphate ion.
The following steps are involved in the manufacturing of potassium dichromate:
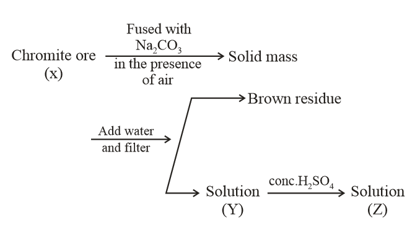
What is the difference in the oxidation number of between X and Y?
In which reaction no colour change will be observed?
Which of the following reaction is spontaneous oxidation-reduction reaction?
